Oxidation Number of Carbon
The oxidation number of iónes monatomic is equal to the charge of these ions. Bonds between atoms of the same element homonuclear bonds are always divided equally.
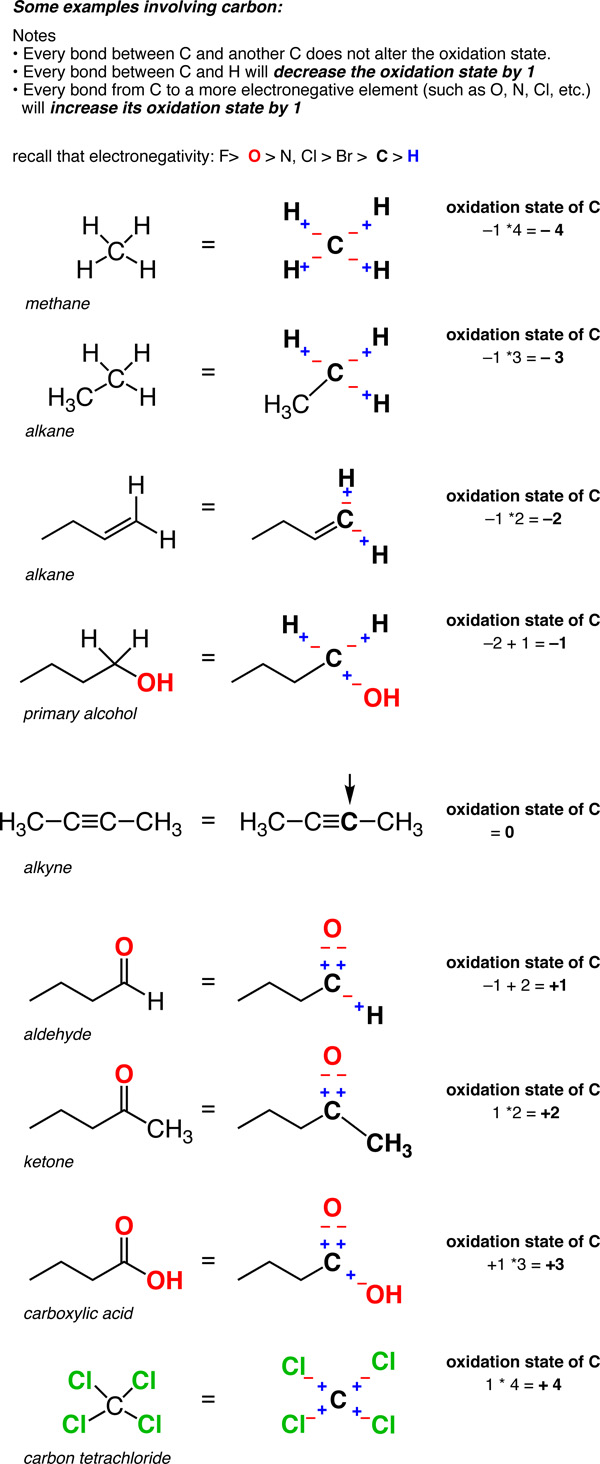
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
The hydrogen atom has one bond to carbon 1 for an oxidation number of 1.

. The structure of H C N is H C N. Thirdly remember that oxidation state is calculated based on the heteronuclear bonds. The oxidation state of carbon in COCl2 is 4 because it shared two of its valence electrons to an oxygen and another two electrons to two chlorine atom.
Thus we can assume that the oxidation number of a carbon atom in a Cyanide ion CN is 2. The oxidation state of OxygenO -2. We have the formula K2CO3 Oxidation number of K 1 x 2 atoms of K 2 Oxidation number of O 2 x 3 atoms of O 6 Principle of electrical neutrality applies in this sense whereby pure ionic substances are electrically neutral.
Answer 1 of 4. Second look at what it is bonded to 3 hydrogens and one carbon. Using the structure method 1 a formal charge is assumed assigning the bonding electrons to the most electronegative atom.
Cl -1. The oxidation number of the hydrogen is 1 in all its compounds except in the metal hydrides that is -1. CH4 and diamond respectively are A3 4 and 4 B3 4 and zero C6 4 and zero D6 4 and 4.
Na 1 Ca 2 2. H 2 O 2 P 4 etc 0. First pick your atom in this case carbon.
The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. The carbon atom has one bond to hydrogen -1 and three bonds to nitrogen 3 for an oxidation number of 2. Calculating the oxidation number of CarbonC in Carbon suboxide C 3 O 2.
Therefore the overall oxidation number for chlorine in HClO₄ is VI½. Therefore in a net overall charge of zero for a compound X 2- 2 1- 0 X must equal 4 the oxidation number of carbon. Formula of acetic acid ethanoic acid is CH 3 COOH meaning the molecular formula is C 2 H 4 O 2.
Change in oxidation state of carbon is from 4 4 to 4 4. Let each carbon atom be X. R is an abbreviation for any group in.
So oxidation number of. N a C X 2 H. Answer 1 of 3.
If two or more than two atoms of an element are present in the moleculeion such as Na 2 S 2 O 3 the oxidation number of the atom of that element will then be the average of the oxidation number of all the atoms of that element. Why is the oxidation number of carbon in acetic acid methanoic acid -2. Therefore 2X 41 2-2 0 2X 4 - 4 0 2X 0 X 0.
As x4 0 x 4 x 4 0 x 4. Similarly when calculating the oxidation number of carbon in CH₃CHO we must take into account that there are two carbons with identical bonding. Different ways of displaying oxidation numbers of ethanol and acetic acid.
Oxygen most often has a charge of 2- and chloride most usually 1-. Each O is -2 and each H is 1. The oxidation number of an element in its free state is zero 0.
The nitrogen atom has three bonds to carbon -3 for an oxidation number of -3. How do you work this out when there are two carbon atoms present. Fourthly use a periodic table of elements to decide which element of.
The oxidation number of a Carbon atom and the oxidation number of a Nitrogen atom is equal to the charge of the Cyanide ion. This Demonstration shows two ways of assigning an oxidation number to a carbon atom in an organic compound. Bonding between atoms of the same species produces no formal charge difference.

Oxidation Number Example 3 Redox Reactions Oxidation Chemistry

Oxidation Number How To Find Oxidation State

Oxidation Number Easy Science Easy Science Oxidation Education Subjects

Oxidation Number State Definition Rules How To Find And Examples
No comments for "Oxidation Number of Carbon"
Post a Comment